Ferrocene NHS Ester |Syn: Ferrocene Carboxylic N-hydroxysuccinimide Ester | Part No. HPT1002
 Click to enlarge |
|
|
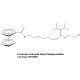
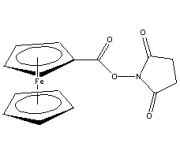
Ferrocene NHS Ester
(Ferrocene Carboxylic N-hydroxysuccinimide Ester)
Highlights:
Ferrocene-NHS Ester Specifications:
Chemical Name: Ferrocene carboxylic N-hydroxysuccinimide ester
Formula: C15H13FeNO4
MW: 327.11
Purity: > 97%
Absorbance: Lamdamax: 438 nm, Extinction Coefficient: 230 M-1 cm-1
Appearance: Yellow solid
Solubility: DMSO, Acetonitrile
Storage: RT, desiccate. Ships at ambient temperature
*Convenient Reaction Pack: Five vials with 10 mg Ferrocene NHS Ester per vial: Cost: $450.00
Please see "Available Options" menu above to order.
* Related Ferrocene Modifying Reagents Offered by Fivephoton Biochemicals
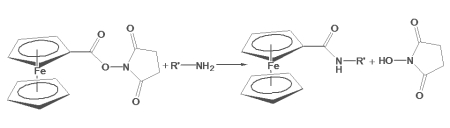
Ferrocene-NHS Ester is an electrochemical active reporter molecule that reacts with primary amines in peptides, proteins and oligonucleotides. The NHS reactive group allows for conjugation to both alpha and epsilon amino groups in peptide chains, and to free thiol and amines in modified nucleotides. Conjugation of ferrocene-NHS ester to the epsilon-amino group in lysine provides a multi-carbon linker between the polypeptide backbone and the ferrocene reporter, which limits steric hindrance on intermolecular interactions. Below is a schematic diagram revealing the reaction between Ferrocene-NHS ester and the epsilon-amino group in lysine, outlining the method of conjugation to peptide chains. A similar scheme applies toward conjugating to modified oligonucleotides. Conjugated ferrocene is detected with voltammetry.
Fig. 1. General Reaction scheme of ferrocene carboxylic NHS Ester with primary amines on biomolecules, forming an amide linkage in the conjugated product:
Examples of applications of ferrocene conjugates as a molecular reporter for peptides and proteins:
1. Conjugation of ferrocene to antibodies to detect VEGF in serum using voltammetry.1
2. Detector of protease activity using ferrocene labeled substrate.2
3. Binding of enzymes to peptide substrates that were conjugated with ferrocene.
References applying Ferrocene as a molecular reporter on peptides:
Protocol: Peptide Conjugation
Suspend the peptide (or protein) into a non-amine buffer at pH 8.3 (for example 10 mM Hepes, 150 mM NaCl, pH 8.3) to the highest concentration that the peptide maintains solubility. Dissolve Ferrocene NHS Ester in DMSO at 100mg/ml, then immediately dispense into the peptide-buffer solution at a 100-1000X molar excess of Ferrocene Ester relative to peptide. Let the reaction proceed at RT with gentle shaking for 4 hrs. (If you are conjugating a protein in a cell lysate, use non-amine protease inhibitors and perform the reaction overnight at 4oC with gentle shaking). Purify the protein-ferrocene conjugate using gel filtration, or desalt in a spin column, collecting the ferrocene-peptide in the void volume. The Ferrocene-linked peptide will attain a yellowish coloration. The peptide-ferrocene conjugate can be followed at 438 nm in correspondence with the peptide bond absorbance peak.
Protocol: Oligonucleotide Conjugation
Ferrocene-NHS Ester (9.85 mg, 30.11 micromol) is dissolved in 1.0 milliliter of methyl sulfoxide and 3 micromole amino modified oligonucleotide is dissolved in 800 microliter of 0.2 M sodium carbonate buffer (pH 9.5). The ester solution (400 microliter) is added to the amino-oligonucleotide solution. The mixture is left for 16 hour at 4oC, after which it is chromatographed on a Sephadex G-25 column using de-ionized water/carbonate buffer (50/50) as eluent. The fraction with yellow color is dialyzed against water to remove excess salts and unreacted reagents, and then freeze-dried. The final product is stored in the refrigerator until use.
Oligonucleotide Synthesis With Ferrocene Modification Scheme

The following provides examples for measuring ferrocene derivatives
Product Citations
Rudewicz-Kowalczyk, Daria, and Iwona Grabowska. (2022). "Antibody–Ferrocene Conjugates as a Platform for Electro-Chemical Detection of Low-Density Lipoprotein." Molecules 27.17 (2022): 5492. L Kashefi-Kheyrabadi, HV Nguyen, A Go, et. Al. (2022). Rapid, multiplexed, and nucleic acid amplification-free detection of SARS-CoV-2 RNA using an electrochemical biosensor. Biosensors and Bioelectron (195), 1 January 2022, 113649 click here Jagotamoy Das et al , (2021). Reagentless biomolecular analysis using a molecular pendulum. Nature Chemistry. 13, 428–434 click here Ribeiro, W. C., L. M. Gonçalves, S. Liébana, M. I. Pividori, and P. R. Bueno. "Molecular conductance of double-stranded DNA evaluated by electrochemical capacitance spectroscopy." Nanoscale (2016). Link to article: click here Link to methods supplement: click here
References on Applying Ferrocene as a Molecular Reporter on Oligonucleotides, and Voltammatry Applications:
Storage and Handling Ferrocene-NHS Ester: Desiccate, RT.
Safety: Harmful. Avoid ingestion, skin and eye contact.
Shipping: Ships at ambient temperature. Availalbe for international shipping.
kw. electrochemical oligonucleotide modification conjugate, ferrocene conjugate, electrochemical modification reagent,
|
| Category | Download Link |
| Specification Sheet | click here |
|
Conjugation Protocol |
click here |
| COA |
click here |
| MSDS | click here |
 Products
Products Manuals
Manuals