Immunofluorescence Labeling Kit (INF-1)
 Click to enlarge |
|
IMMUNOFLUORESCENCE LABELING KIT
Highlights
-
For single and multiple immunofluorescent labeling (i.e. labeling with 2-3 fluorescent color tags simultaneously).
- The protocol enables detection of antigens in intraellular and extracellular locations.
- Contains saponin for membrane permeabilization.
-
Enables detection of cellular organelles such as Golgi, nuclei, mitochondria, endoplasmic reticulum as well as cell surface proteins.
- Stock solutions can be thawed-frozen multiple times, or aliquoted and frozen.
Immunofluorescence Kit Contents
-
Instruction Manual
-
10X PBS pH 7.4; 16 ml
-
10X Quenching Buffer; 25 ml
-
10X Permeabilization Buffer; 8 ml
-
10X Intracellular Detection Buffer; 60 ml
-
Forceps set
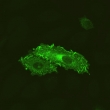
The immunofluorescence labeling kit employs saponin to permeabilize membranes. Saponin more effectively preserves membrane structure, enhancing resolution of intracellular organelles in fluorescent microscopy images.
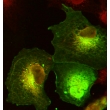
Along with intracellular antigens, transmembrane and cell surface proteins can also be visualized with immunofluorescence protocols that employ membrane permeabilization since antibodies are accessible to all cellular compartments and locations.
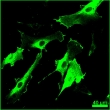
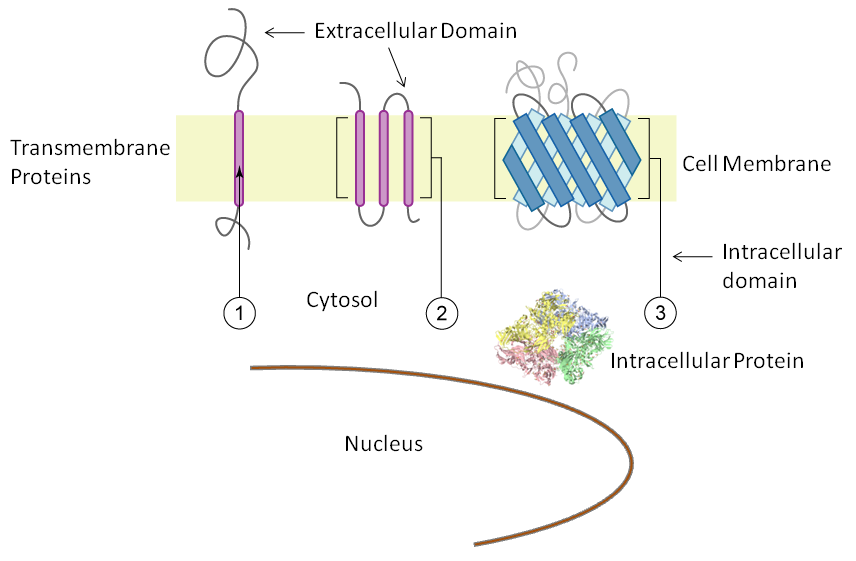
Cellular Regions and Membrane Topology. This immunofluorescence labeling kit labels proteins in all regions and organelles. Use a domain selective antibody to exclusively label extracellular or intracellular domains.
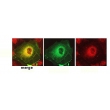
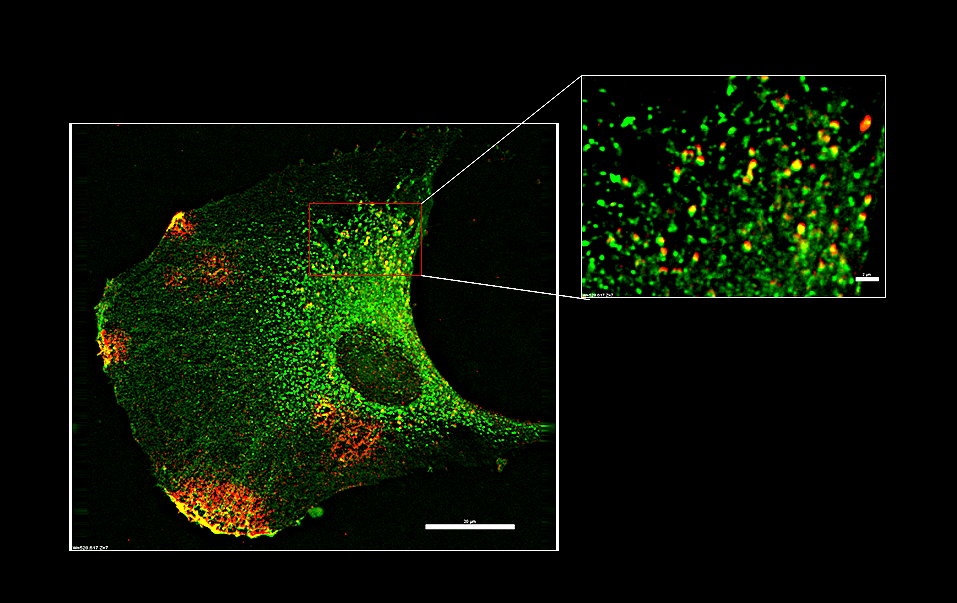
Example Images
- Blot V and McGraw TE. Use of quantitative immunofluorescence microscopy to study intracellular trafficking: studies of the GLUT4 glucose transporter. Methods Mol Biol 457: 347-366, 2008.
-
Constans J, Oksman F, and Viau M. Binding of the apo and holo forms of the serum vitamin D-binding protein to human lymphocyte cytoplasm and membrane by indirect immunofluorescence. Immunol Lett 3: 159-162, 1981.
-
Crivellato E, Travan L, Candussio L, Bartoli Klugmann F, and Decorti G. Identification of P-glycoprotein at the membrane of mast cell secretory granules. An immunofluorescence and protein A-gold electron microscopical investigation. Histochem J 29: 193-198, 1997.
- Doody A and Putnam D. Identification of compartments involved in mammalian subcellular trafficking pathways by indirect immunofluorescence. Methods Mol Med 127: 127-136, 2006.
-
Fleischmajer R, Dessau W, Timpl R, Krieg T, Luderschmidt C, and Wiestner M. Immunofluorescence analysis of collagen, fibronectin, and basement membrane protein in scleroderma skin. J Invest Dermatol 75: 270-274, 1980.
-
Gajl-Peczalska K. Plasma Protein Composition Of Hyaline Membrane In The Newborn As Studies By Immunofluorescence. Arch Dis Child 39: 226-231, 1964.
-
Lejneva OM, Abelev GI, Dorfman NA, Strand M, and August JT. Localization of a murine oncornavirus 15,000-dalton virion protein on the membrane of neoplastic cells: analysis by immunofluorescence and immunoelectron microscopy. Virology 75: 281-292, 1976.
-
Risau W, Saumweber H, and Symmons P. Monoclonal antibodies against a nuclear membrane protein of Drosophila. Localization by indirect immunofluorescence and detection of antigen using a new protein blotting procedure. Exp Cell Res 133: 47-54, 1981.
-
Tezuka T and Takahashi M. The 55-kd keratohyalin granule protein has the same epitope as the 43-kd stratum corneum membrane protein: immunofluorescence and immunoblotting studies using a monoclonal antibody to the 55-kd keratohyalin granule protein. Arch Dermatol Res 280: 462-468, 1989.
- Wang H, Lee EW, Cai X, Ni Z, Zhou L, and Mao Q. Membrane topology of the human breast cancer resistance protein (BCRP/ABCG2) determined by epitope insertion and immunofluorescence. Biochemistry 47: 13778-13787, 2008.
 Products
Products Manuals
Manuals