|
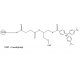
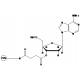
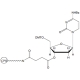
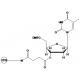
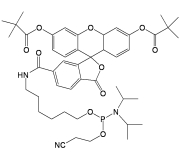
6-FAM Phosphoramidite
6-FAM Phosphoramidite (FAM-amidite; FAM Phosphoramidite) is a fluorescent nucleotide labeling reagent. FIVEphoton Biochemicals offers high purity 6-FAM Phosphoramidite for small to large scale oligonucleotide synthesis. 6-FAM Phosphoramidite has applicability in detection of nucleotide hybridization, changes in nucleotide conformation, transcription factor binding to DNA, tracing of siRNA introduction into cells, quenching interactions between labeled oligonucleotides with interacting binding molecules, and quantitation of PCR amplification.
6-FAM Phosphoramidite: Specifications
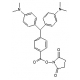
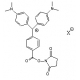
Molecular Formula: C46H58N3O10P
Molecular Weight: 843.94
CAS Number: 204697-37-0
Purity (HPLC): > 95%
Solubility: Insoluble in water
Appearance: White or clear crystals
Spectral: Ex: 494nm. Em 522nm
Storage: -20oC
COA: Contact us for the COA of the current lot.
Available Options for 6-FAM Phosphoramidite.
|
Product Number
|
Quantity
|
Price (USD)
|
|
HPT-1403-25
|
250mg
|
$150.00
|
* Bulk order discounts are available for commercial customers. Please inquire with customersupport@fivephoton.com for pricing.
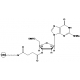
Reaction scheme to label pentose group of oligonucleotide with 6-FAM-Phosphoramidite.

References outlining applications of 6-FAM Phosphoramidite
-
Browne KA. (2005) Sequence-specific, self-reporting hairpin inversion probes. J Am Chem Soc, 127, 1989.
-
Kim SJ, Bang EK, Kwon HJ, Shim JS, Kim BH. (2004) Modified oligonucleotides containing lithocholic acid in their backbones: their enhanced cellular uptake and their mimicking of hairpin structures. Chembiochem, 5, 1517.
-
Dobson N, McDowell DG, French DJ, Brown LJ, Mellor JM, Brown T. (2003) Synthesis of HyBeacons and dual-labelled probes containing 2'-fluorescent groups for use in genetic analysis. Chem Commun (Camb), 1234.
-
Kutyavin IV, Lokhov SG, Afonina IA, Dempcy R, Gall AA, Gorn VV, Lukhtanov E, Metcalf M, Mills A, Reed MW, Sanders S, Shishkina I, Vermeulen NM. (2002) Reduce aggregation and improved specificity of G-rich oligodeoxyribonucleotides containing pyrazolo[3,4-d]pyrimidine guanine bases. Nucleic Acids Res, 30, 4952.
-
Hill KW, Taunton-Rigby J, Carter JD, Kropp E, Vagle K, Pieken W, McGee DP, Husar GM, Leuck M, Anziano DJ, Sebesta DP. (2001) Diels--Alder bioconjugation of dienemodified oligonucleotides. J Org Chem, 66, 5352.
-
Wu H, Skrzypczynski Z, Cornwell MJ, Aboleneen H. (2000) Identification of unexpected modifications of fluorescein-labeled oligodeoxynucleotides by nuclease P1 digestion and
-
mass spectrometric techniques. Rapid Commun Mass Spectrom, 14, 26.
-
Hakala H, Virta P, Salo H, Lonnberg H. (1998) Simultaneous detection of several oligonucleotides by time-resolved fluorometry: the use of a mixture of categorized microparticles in a sandwich type mixed-phase hybridization assay. Nucleic Acids Res, 26, 5581.
-
Ramirez-Vick JE, Garcia AA, Lee J. (1998) Recovery of an oligonucleotide using silver ions immobilized onto paramagnetic particles. Prep Biochem Biotechnol, 28, 243.
-
Meyer KL, Hanna MM. (1996) Synthesis and characterization of a new 5-thiol-protected deoxyuridine phosphoramidite for site-specific modification of DNA. Bioconjug Chem, 7, 401.
-
Hagmar P, Bailey M, Tong G, Haralambidis J, Sawyer WH, Davidson BE. (1995). Synthesis and characterisation of fluorescent oligonucleotides. Effect of internal labelling on protein recognition. Biochim Biophys Acta, 1244, 259.
-
Lee SP, Censullo ML, Kim HG, Knutson JR, Han MK. (1995) Characterization of endonucleolytic activity of HIV-1 integrase using a fluorogenic substrate. Anal Biochem,
-
227, 295.
-
Nelson PS, Kent M, Muthini S. (1992) Oligonucleotide labeling methods. 3. Direct labeling of oligonucleotides employing a novel, non-nucleosidic, 2-aminobutyl-1,3-propanediol backbone. Nucleic Acids Res, 20, 6253.
-
Schubert F, Cech D, Reinhardt R, Wiesner P. (1992) Fluorescent labelling of sequencing primers for automated oligonucleotide synthesis. DNA Seq, 2, 273.
-
Theisen P, McCollum C, Andrus A. (1992) Fluorescent dye phosphoramidite labelling of oligonucleotides. Nucleic Acids Symp Ser, 99.
Safety: Irritant. Avoid contact and inhalation.
Storage: -20oC. Avoid light exposure. Shipped at ambient temperature.
Shipping Options: Overnight, and two-day Fedex. International delivery is available on this item.
|

 Products
Products Manuals
Manuals